The Application Gallery features COMSOL Multiphysics® tutorial and demo app files pertinent to the electrical, structural, acoustics, fluid, heat, and chemical disciplines. You can use these examples as a starting point for your own simulation work by downloading the tutorial model or demo app file and its accompanying instructions.
Search for tutorials and apps relevant to your area of expertise via the Quick Search feature. Note that many of the examples featured here can also be accessed via the Application Libraries that are built into the COMSOL Multiphysics® software and available from the File menu.
Separation Through Dialysis
Dialysis is a widely used chemical species separation method. One such example is hemodialysis, which acts as artificial kidneys for people with renal failure. In dialysis, only specific components are allowed to diffuse through the membrane, based on differences in molecular size and ... Read More
Liquid–Liquid Extraction
Liquid-liquid extraction is a process used to separate or transfer species between two immiscible liquids. Transfer of species from one phase to the other is driven by a difference in relative solubility. In this model a water filled extraction column is studied. Oil droplets containing ... Read More
Hydrodealkylation in a Membrane Reactor
This application demonstrates how you can access and include external thermodynamic and physical property calculations in your simulation, using the Thermodynamics feature. The example shows how you can easily modify the predefined Plug-flow reactor type in the Reaction Engineering ... Read More
Heterogeneous Model of a Porous Catalyst Bed
A catalyst particle with a hypothetical microstructure is described in detail. The heterogeneous description is approximated in a second model with a homogeneous particle and the results from the two approaches are compared. See: https://www.comsol.se/blogs/modeling-approaches-in ... Read More
Dimensioning a Distillation Column for Separation of Water and Ethanol
This example shows how to make a simple model for a binary distillation process. A thermodynamics property package, available in the Chemical Reaction Engineering Module, is created to study the separation of a nonideal mixture of ethanol and water. The required equilibrium relationship ... Read More
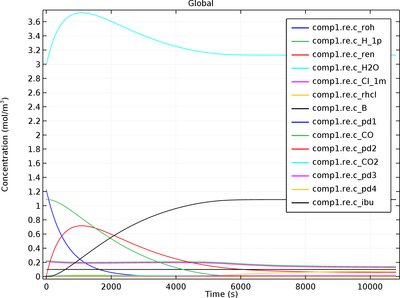
Ibuprofen Synthesis
Kinetic analysis of catalytic reactions is essential for understanding rate behavior as well as the reaction mechanism. Developing knowledge of intrinsic reaction kinetics and of rate equations is central to reaction engineering studies aimed at improving reactor design. This model ... Read More
Transport in an Electrokinetic Valve
This application presents an example of pressure driven flow and electrophoresis in a 3D micro channel system. Researchers often use a device similar to the one in this model as an electrokinetic sample injector in biochips to obtain well-defined sample volumes of dissociated acids and ... Read More
Precipitation of Barium Sulfate
Crystallization is an important separation process in the chemical industry. It is used for the production of pharmaceuticals and industrial chemicals. It can also be used in resource recovery as a way of separating valuable materials from waste. This model solves a discretized ... Read More
Fine Chemical Production in a Plate Reactor
Plate reactors running under continuous conditions have emerged as candidates to replace batch reactors, primarily in fine chemicals and pharmaceuticals production. One of the advantages of the plate reactor design is that it allows for efficient temperature control of the reacting ... Read More
Cubic Autocatalysis: Exploring the Gray–Scott Model
A deceptively simple system of two species and two reactions, describing the autocatalytic conversion of a substance, is shown to display a surprisingly exotic behavior. Starting from a 0D CSTR description, a 2D reaction-diffusion problem is formulated showing rich patterns stemming from ... Read More