Modeling the Catalytic Conversion of Steel Mill Gases Using the Example of Methanol Synthesis
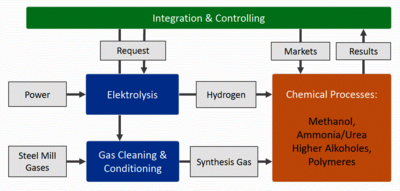
The conversion of steel mill off-gases to base chemicals is a promising perspective to improve the financial return of steelmaking in Europe and fight CO2 emissions. In the Carbon2Chem® joint research project, mathematical models are developed to simulate operation scenarios of cross-industrial networks and chemical plants embedded in a steel mill environment. Methodically, the modeling follows a multilevel approach combining models of different depth and origin in a common simulation framework. Chemical processing of steel mill gases can be fed by three main carbon sources of steel processing: • Coke oven gas (COG): hydrogen and methane rich • Blast furnace gas (BFG): carbon and nitrogen rich • Basic oxygen furnace gas (BOFG): carbon rich At present, in a modern steel mill all these gases are part of an integrated energy concept. Within the Carbon2Chem® crossover concept, certain gas contingents should be used as sources for several chemical processes while other parts are integrated in steel processing. Chemical routes considered in the project are: • Methanol synthesis • Ammonia synthesis • Synthesis of higher alcohols • Polymer synthesis In Fig. 1, the general basic flowsheet of an integrated methanol process is shown. It starts from the steel mill H1 with the three gas types COG, BFG and BOFG. The gases are processed in three separate lines consisting of splitters (SP1, SP2, SP3) defining the gas fraction used for further chemical processing, optional buffer tanks (B3, B4, B5), and gas cleaning and conditioning units (S1, S2, S3). The second operation line is the hydrogen supply with the water electrolysis unit (E1) and an optional hydrogen storage tank (B2). The final synthesis gas is mixed in a buffer tank (B1) and fed to the methanol synthesis plant (R1). Reactor models for the heterogeneous catalytic conversion of synthesis gases are set up as time-dependent models within COMSOL Multiphysics® at three different levels (0-d, 1-d, 2-d). The mathematics interface and the chemical reaction engineering module are used to define the resulting systems of partial differential equation. Other elements of the flowsheet are specified as 0-d differential/algebraic models and linked in COMSOL® by appropriate coupling operators. So high level reactor models and low level models for further process components are linked in a flowsheet simulation consisting of more than 30 COMSOL® components demonstrating the coupling capabilities of the software. The synthesis of methanol from steel mill gases is simulated and analyzed for several different gas conditioning scenarios in stationary and time dependent studies. In the presentation the main challenges of producing chemicals from steel mill gases are shown and explained from meaningful simulation results. A second aspect of the work is the parallel application of low level and high level reactor models in chemical process simulation and the suitability of COMSOL Multiphysics® for process simulation tasks.
Download
- schlüter_presentation.pdf - 1.75MB
- schlüter_abstract.pdf - 0.07MB
