Acid-Base Equilibria and Copper Speciation in Ammonia Solution
Application ID: 103951
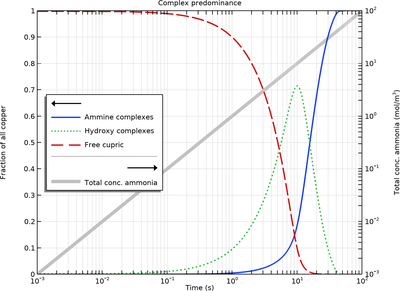
Cupric ions show a strong affinity to ammonia in aqueous solutions, forming strongly colored deep blue complexes. The relative amounts of the different ammine ligand complexes, with varying coordination numbers, are governed by the stability constants of the equilibria forming the coordination compounds. By entering a series of equilibrium reactions, and an external source of ammonia, the Time Dependent study can be used to produce a speciation diagram, which shows how the concentration of the different complexes vary with the total concentration of added ammonia.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Chemical Reaction Engineering Module, or Electric Discharge Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.
