Modelling and Simulating CO2 Electro-Reduction to Formic Acid using MEC- Microfluidic Electrolytic Cells
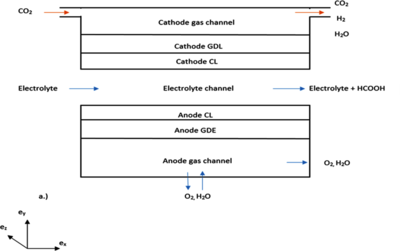
Model calibration and validation were implemented using experimental data from literature. We utilized a microfluidic electrochemical cell that was configured with parallel, rectangular, and multi-layered channels, which is operating in a co-flow mode. CO2 gas stream enters the cathode gas channel, while the anode is open to the atmosphere. Ionic liquid electrolyte [EMIM][BF4] or [EMIM][CF3COOCH3] then flows through the electrolyte channel between two gas diffusion electrodes (GDEs). The cathode is coated with a Bi-Sn catalyst, while the anode is coated with Pt blank. After which, a graphite current-collector is connected to each GDE at the other side.
We employed the Battery & Fuel cell module, inputted necessary material, defined the domains, boundaries, and the physics, coupled and meshed the model. In detail, the full set of governing equations together with boundary conditions, electrochemical reaction kinetic equations and other constitutive equations were solved numerically with COMSOL Multiphysics® using finite element method for the spatial discretization. Further detail, the model was implemented in COMSOL Multiphysics® 5.3a using base case parameters and solved using the direct solver, PARDISO, with a relative convergence tolerance of 10-6, through a fully coupled approach. A coarse mesh of about 2500 elements for the cell was first used. Consecutive mesh adaptation of up to 120000 elements allowed for high resolution in the GDEs. Mesh independence was ensured by ensuring that successive mesh adaptations did not change CO2 conversion by more than 0.1%. One simulation takes 3-8 minutes on an Intel® Core™ i5-3770 CPU @ 3.40 GHz PC with 8.0 GB RAM. The model integrates charge and species transport, mass conservation, and momentum with electrochemistry. Specifically, a comparison between the influence of [EMIM][BF4] 1-ethyl-3-methyl imidazolium tetra-fluoroborate and [EMIM][CF3COOCH3] 1-ethyl-3-methylimidazolium tri-fluoroacetate ionic liquid electrolytes on the CD, FE and CO2 conversion are studied. Based on concentration contours; mass transfer characteristics analysis, this simulation is carried out at a constant mole ratio of 0.5:0.5 of Bi-Sn catalyst with 85% v/v. electrolyte concentration.
After validating with experimental data from literature, further simulations reveal performance measures (current density, faradaic efficiency and CO2 conversion). Employing [EMIM][CF3COOCH3] electrolyte, increasing the negative cathode potential results in higher HCOOH current density, and H2 current density. However, [EMIM][BF4] compared to [EMIM][CF3COOCH3] electrolyte displays a slightly higher HCOOH current density (+2 mA/cm2) and CO2 conversion. However, [EMIM][CF3COOCH3] electrolyte displayed a higher faradaic efficiency and CO2 conversion.
This model achieved a CD-Current Density (~58 mA/cm2), FE-Faradaic Efficiency (~99%) and CO2 conversion (82%) in the electrochemical reduction of CO2 to formic acid. No parametric studies are carried out.
Download
- Cambridge_2019_Poster_Template.pptx - 0.35MB
