The Application Gallery features COMSOL Multiphysics® tutorial and demo app files pertinent to the electrical, structural, acoustics, fluid, heat, and chemical disciplines. You can use these examples as a starting point for your own simulation work by downloading the tutorial model or demo app file and its accompanying instructions.
Search for tutorials and apps relevant to your area of expertise via the Quick Search feature. Note that many of the examples featured here can also be accessed via the Application Libraries that are built into the COMSOL Multiphysics® software and available from the File menu.
This example applies the Electrophoretic Transport and Laminar Flow interfaces to model isoelectric separation in a free-flow electrophoresis device. A stream containing six different ionic species is shown to be divided into pure component streams by means of migrative transport in an ... Read More
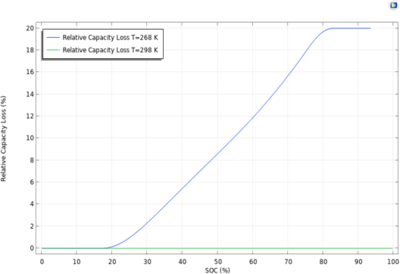
Deposition of metallic lithium on the negative electrode in preference to lithium intercalation is known to be a capacity loss and safety concern for lithium-ion batteries. Harsh charge conditions such as high currents (fast charging) and/or low temperatures can lead to lithium plating. ... Read More
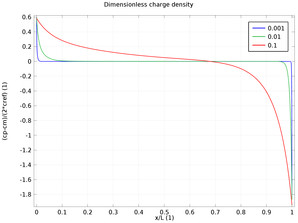
In the diffuse double layer and within the first few nanometers of an electrode surface, the assumption of electroneutrality is not valid due to charge separation. Typically, the diffuse double layer may be of interest when modeling very thin layers of electrolyte including those in ... Read More
The copper current collector on negative graphite electrodes in lithium-ion batteries have been seen to dissolve at over discharge. This can be a safety concern as the dissolution damages the current collector irreversibly and dissolved copper ions can redeposit and form dendrites. ... Read More
Alkaline water electrolysis is a well-established industrial process for producing hydrogen gas. In the cell, hydrogen gas is formed at the cathode whereas oxygen gas is formed at the anode. The electrolyte is an aqueous liquid, and when the evolved gases form bubbles, the effective ... Read More
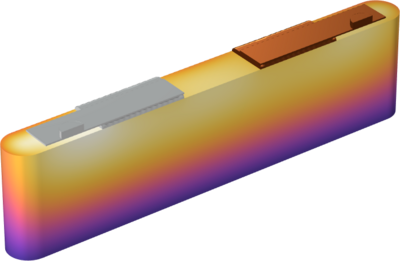
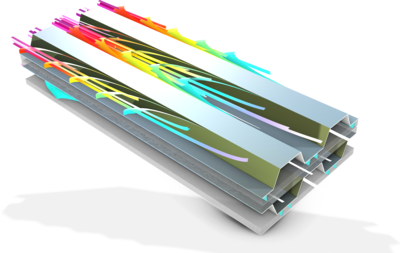
This model exemplifies how to compute the internal temperature distribution in a prismatic battery during a high-rate charge. The electrochemistry is described by a a lumped two-electrode model, which is coupled to the heat transfer model. The heat transfer model includes the effects of ... Read More
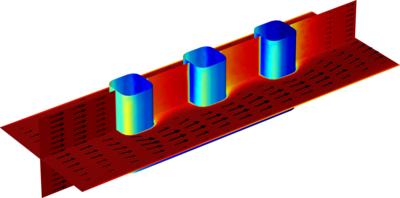
The electrochemical cell shown in this model can be regarded as a unit cell of a larger wire-mesh electrode that is common in many industrial processes. One of the most important aspects in the design of electrochemical cells is the current density distributions in the electrolyte and ... Read More
Electrochemical supercapacitors feature relatively higher energy densities than conventional capacitors. With several advantages, such as fast charging, long charge–discharge cycles, and broad operating temperature ranges, electrochemical supercapacitors have found wide applications in ... Read More
This model defines a zero-gap alkaline water electrolyzer, where oxygen and hydrogen gas are evolved in porous gas diffusion nickel felt electrodes, placed adjacent to a porous separator (diaphragm). The geometry defines a unit cell of an electrolyzer stack, in turn comprising two full ... Read More
Some positive electrode materials are known to deteriorate in overcharged lithium-ion battery cells. Predominantly, manganese containing electrode materials such as LMO and NMC can loose capacity due to manganese dissolving from the materials at overcharge. This decomposition is a ... Read More