Battery Design Blog Posts
How to Define Load Cycles in Battery Models
Specifying a load profile is critical when modeling a battery system. Explore several approaches for doing so in COMSOL Multiphysics® and the Battery Design Module.
Analyzing Thermal Distribution in a Li-Ion Battery Pack
Deviating from optimal operating temperatures in a Li-ion battery pack can cause performance losses or even failure. Learn how to optimize the power design to control the temperature distribution.
Improving Tabbing Design in Cylindrical Batteries
A major electric vehicle and battery manufacturer announced a new “tabless” design concept for cylindrical lithium-ion batteries. In this blog post, we explore this concept with simulation.
Approaching an Electrochemical Model from Scratch: Lemon Battery
The lemon battery: A high school chemistry experiment, and a great example when learning the general process for how to set up electrochemistry and battery models from scratch.
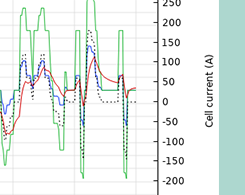
Estimating Parameters for a Li-Ion Battery via a Lumped Model
When performing an electrochemical analysis on a battery, an engineer might not have all of the information from the manufacturer. The solution? Parameter estimation via a lumped model…

Analyzing the Liquid Cooling of a Li-Ion Battery Pack
A sudden temperature increase or decrease can affect the efficiency of a lithium-ion battery. For the battery packs used in electric vehicles and other application areas, this can be a problem…
The 2019 Nobel Prize in Chemistry Celebrates Li-Ion Battery Research
John Goodenough, M. Stanley Whittingham, and Akira Yoshino, winners of the 2019 Nobel Prize in Chemistry, come from different places and researched lithium-ion batteries at different times.
Electrode Balancing of a Lithium-Ion Battery with COMSOL®
Electrode balancing is an important consideration for battery cell engineers. Get an overview and mathematical framework of this phenomenon and learn how to analyze it in a lithium-ion battery.
