To lessen our dependence on fossil fuels, the world must transition toward renewable sources of energy, like wind and solar power. We also must deliver that energy to where it’s needed most. One promising method for energy storage and transport involves the most abundant element in the universe: hydrogen. A polymer electrolyte membrane (PEM) electrolyzer uses electricity to extract hydrogen gas from water. Using the COMSOL Multiphysics® software, you can simulate the operation of a PEM electrolyzer. Improving the efficiency of this device could help make stored hydrogen a viable alternative to electric batteries and liquid fossil fuels.
The Challenges of Wind and Solar Power Generation
Renewable power generation is helping the world move toward a less carbon-intensive economy, but sources like wind and solar bring their own challenges. It can be difficult to balance wind and solar energy production with consumer demand. Also, the optimal locations for wind turbines and solar panels are often in remote areas with limited electrical grid capacity. These conditions make improved energy storage and transport a vital complement to expanded renewable energy production.
Electric batteries are a familiar means of storing energy, but mining the metals used in batteries can bring environmental costs, and disposal of old batteries can also be problematic. While intensive research is focused on improving battery design, the vast scale of future energy storage demand means that other approaches are also needed.
The Potential of Hydrogen-Based Energy Storage
A hydrogen electrolysis-based energy storage system could help address the challenges of decentralized wind and solar power production. Power generation facilities can supply electricity to onsite electrolyzers, which would then use it to separate hydrogen from water. (This process is explained in detail below.) The hydrogen is then captured, stored, and shipped to wherever it’s needed, in tanks or through a pipeline. Electrolytic hydrogen is also required for industrial applications, such as the production of “green steel“.
While this approach has shown promise in tests, the utility sector has not yet made large-scale commitments to the hydrogen electrolysis process. One daunting obstacle is the cost of producing electrolyzers.
Extracting Hydrogen from Water with PEM Electrolyzers
In a PEM electrolyzer cell, two electrode chambers are separated by a polymer membrane. Liquid water is circulated through the anode side. Electrolytic action causes some of the water molecules to split into oxygen gas and hydrogen gas, which move through the membrane and accumulate on the cathode side.
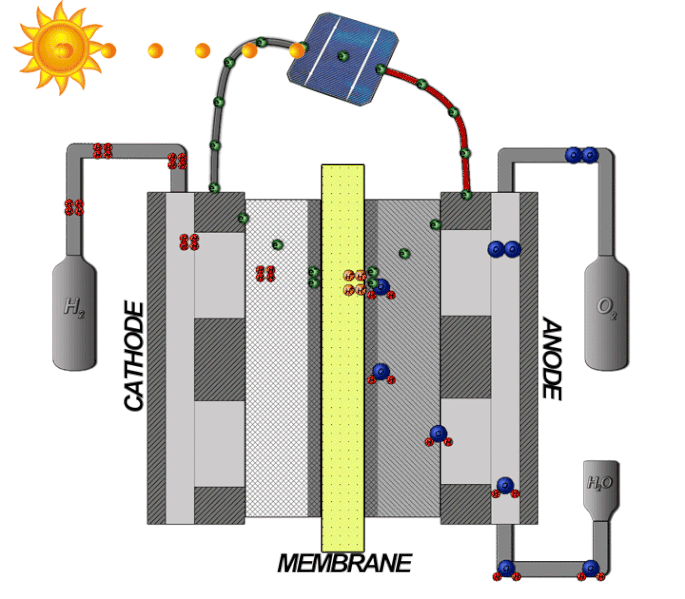
The mechanisms of an electrolyzer. Image by Davidlfritz — Photoshop. Licensed under CC BY-SA 3.0, via Wikimedia Commons.
This electrolysis method provides important benefits, as presented in a 2015 Compendium of Hydrogen Energy report. Compared to other types of electrolyzers, PEM electrolyzers are:
- Compact
- Flexible
- Easily handled
- Tolerant of varying electrical loads
- Able to operate under high-pressure conditions
Despite their promise, PEM electrolyzers have yet to be widely adopted, mostly due to their high initial cost. Their catalytic action requires iridium on the anode side of the device and platinum on the cathode side. While the quantities involved are very small compared to the metals used in batteries, iridium and platinum are some of the rarest metals on Earth. Their high cost means that PEM electrolysis is not yet economically viable. Iridium, in particular, is both expensive and subject to degradation during operation. Therefore, boosting the durability and conversion efficiency of the anode-side iridium layer is an important focus for PEM electrolyzer research.
Simulating Two-Phase Flow to Maximize Conversion Efficiency
The Fuel Cell & Electrolyzer Module includes functionality for modeling a PEM electrolyzer. This type of model enables us to simulate the two-phase fluid dynamics on the anode side of the device, which can help us study its iridium-enabled electrolytic action. We’ll discuss the model and some of its interesting results here, but if you want to jump right into the step-by-step tutorial model, you can find it here: Polymer Electrolyte Membrane Electrolyzer.
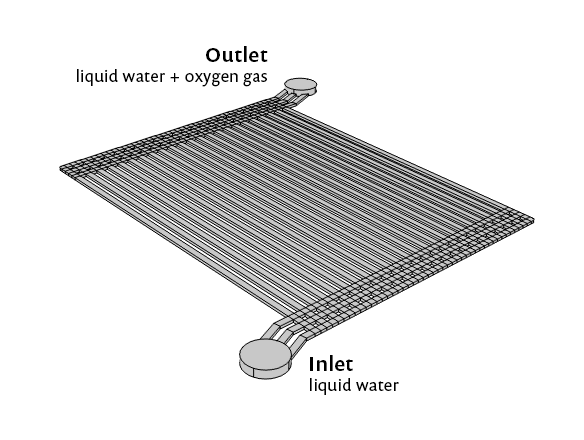
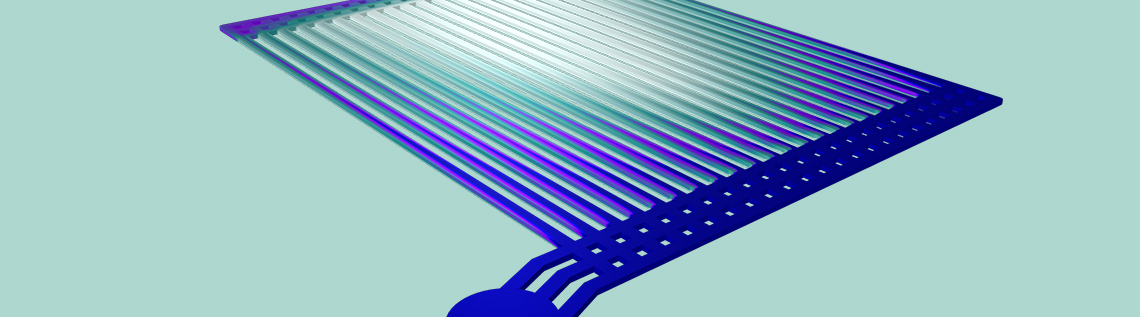
Model geometry for the PEM electrolyzer.
The simulation results demonstrate that, at the end of the electrode flow channels near the center of the device, the gas volume fraction approaches 100%. At the same time, much less gas conversion has occurred in the channel at the far right. Any liquid water that exits the device should have been oxidized, to release protons available for reduction on the cathode side of the electrolyzer. Conversely, the iridium in the large “red zone” is having very little effect, as there is almost no liquid water left for it to oxidize in those channels. This suggests the potential for redesigning the electrolyzer geometry to achieve a more efficient utilization of the catalytic material.
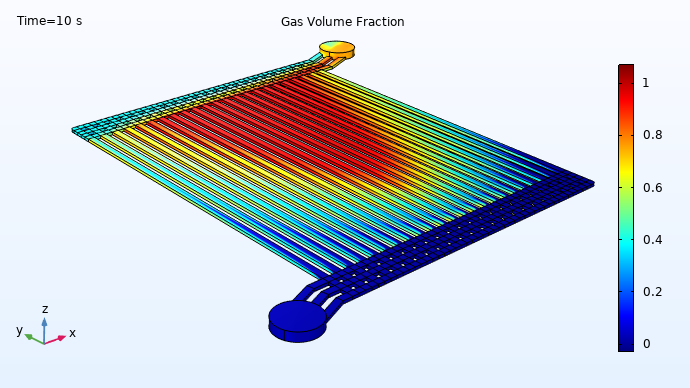
Distribution of liquid water (blue) and emerging oxygen gas (red) during operation of a PEM electrolyzer.
By highlighting potential improvements to the PEM electrolyzer design, simulation can help designers make electrolyzers more efficient — and hydrogen-based energy distribution more viable.
Next Steps
Try simulating two-phase flow in a polymer electrolyte membrane electrolyzer by clicking the button below:




Comments (4)
Abimbola Ashaju
October 29, 2021Hi Alan in the comsol model on alkaline water electrolysis, could you clarify (preferably with reference) what relations/formula was used in specifying the inlet velocity at the anodic and cathodic compartment?
Alan Petrillo
November 1, 2021 COMSOL EmployeeHello Abimbola, thanks for your question. The model is based on the following paper:
J. Nie and Y. Chen, Numerical modeling of three-dimensional two-phase gas-liquid flow in the field plate of a PEM electrolysis cell, Int. J. Hydrogen Energy 35 (2010)3183-3197
The rationale behind the flow rates can be found there.
As for specifying the inlet velocities, that was done by using Inlet features. To better understand them, you can consult documentation or connect with us at the Support Center:
https://www.comsol.com/support
Ramakant Gadhewal
October 18, 2024Hi Alan
Would you be able to share a full-scale model of a PEM electrolyzer ?
JIAWEI HUO
January 20, 2026Hi Alan! The comsol model is base on contextual data. In my research, I need to build a model based on EHP. Would you share the actual size of the comsol model so that I could adapt my parameters?