
There were many interesting posters at this year’s COMSOL Conference in Boston. A couple that caught my eye involved microwave heating and chemical applications. One of them showcases the use of microwave irradiation to speed up chemical reactions. Another — one of the recipients of the Best Poster award — used simulations to optimize their microreactor design with respect to microwave propagation.
The Effect of Microwave Heating on Chemical Reactions
In a poster submitted to this year’s COMSOL Conference in Boston, researchers S. Fujii, H. Kujirai, D. Mochizuki, M. Maitani, E. Suzuki, and Y. Wada showed how they were able to improve the reproducibility of chemical reactions with microwave irradiation as compared to an electric furnace. The use of microwave heating is a rather unusual approach to promoting chemical reactions, one most chemical engineers might not even think of. Indeed, in their introduction, they mention that “it is often pointed out that microwave irradiated reactions have a very low reproducibility.” To prove this wrong, the research group went on to develop a solid-state microwave high-power amplifier (HPA) module with an ultra-precise oscillator, as well as an elliptical applicator.
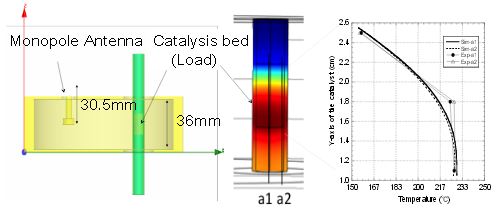
Elliptical applicator simulations. Image by S. Fujii, H. Kujirai, D. Mochizuki, M. Maitani, E. Suzuki, and Y. Wada, and taken from their poster submission.
Since they knew that reaction temperature plays a key role in the chemical reaction rate, the group used COMSOL Multiphysics to determine if there were any hot spots in the applicator (see figure above). They did this by modeling the elliptical applicator and then running electromagnetic wave and heat transfer simulations. Finally, they set up an experiment where they generated hydrogen from the decomposition of methanol first via microwave irradiation and then by way of an electric furnace. As you can see in their results below, the chemical reaction rate was actually three times higher under microwave irradiation.
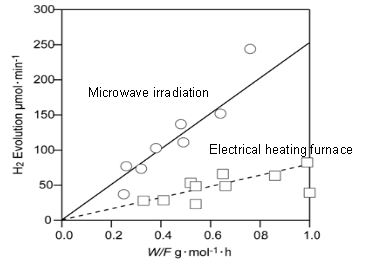
Hydrogen generation by decomposition of methanol. Image by S. Fujii, H. Kujirai, D. Mochizuki, M. Maitani, E. Suzuki, and Y. Wada.
Propagating Microwaves through a Chemical Reactor
While the first group presented research on the effect microwave heating has on chemical reactions, one of the Best Poster winners from the Boston conference detailed research on the ideal conditions for propagating microwaves through a microreactor. Wen-Hsuan Lee and Klavs F. Jensen of Massachusetts Institute of Technology (MIT) developed a design they hoped would allow for organic synthesis:
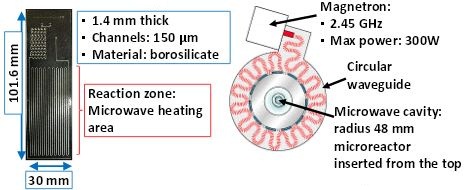
The original design of the microreactor (left) and the top view of the microwave unit (right) that houses the chemical reactor. Image by Wen-Hsuan Lee and Klavs F. Jensen and taken from their poster submission.
When they ran an analysis of the reactor’s heating profile, Lee and Jensen discovered some issues with the original design. Not only was the temperature distribution across the reactor uneven, but the maximum steady-state temperatures were not high enough for the chemical reactions to take place. In order to optimize their design, the researchers turned to COMSOL Multiphysics, the RF Module, and the Heat Transfer Module.
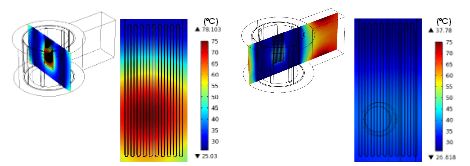
The surface temperature of the microreactor changes with position. Image by Wen-Hsuan Lee and Klavs F. Jensen.
Via simulations, they figured out what was causing the heating issues: low electric field strength. Apparently, the electric field changes depending on both the position and size of the microreactor. Therefore, a higher electric field would solve the heating problems, which the two researchers propose can be achieved by altering the thickness of the reactor.
Download the Posters
If you want to get more details on the results, check out both of the posters using the links below:




Comments (0)