The Application Gallery features COMSOL Multiphysics® tutorial and demo app files pertinent to the electrical, structural, acoustics, fluid, heat, and chemical disciplines. You can use these examples as a starting point for your own simulation work by downloading the tutorial model or demo app file and its accompanying instructions.
Search for tutorials and apps relevant to your area of expertise via the Quick Search feature. Note that many of the examples featured here can also be accessed via the Application Libraries that are built into the COMSOL Multiphysics® software and available from the File menu.
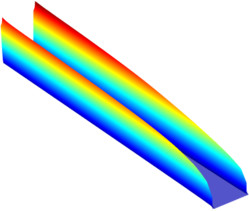
The electrochemical cell shown in this model can be regarded as a unit cell of a larger wire-mesh electrode that is common in many industrial processes. One of the most important aspects in the design of electrochemical cells is the current density distributions in the electrolyte and ... Read More
Due to the large differences in length scales in a lithium-ion battery, with the thickness of the different layers typically being several orders of magnitude smaller than the extension in the sheet direction, a lithium-ion battery is often well represented by a one-dimensional model. ... Read More
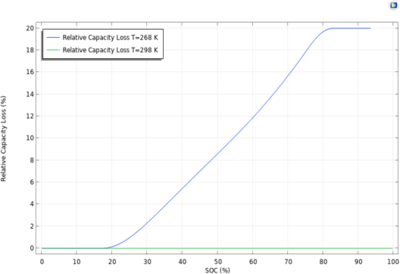
Deposition of metallic lithium on the negative electrode in preference to lithium intercalation is known to be a capacity loss and safety concern for lithium-ion batteries. Harsh charge conditions such as high currents (fast charging) and/or low temperatures can lead to lithium plating. ... Read More
Battery electrodes featuring large heterogeneities in terms of particle sizes may sometimes not be adequately described by homogenized models using one single particle size only. As an alternative to adding multiple instances of the Additional Porous Electrode material node, this ... Read More
In a redox flow battery electrochemical energy is stored as redox couples in the electrolyte, which is stored in tanks outside the electrochemical cell. During operation, electrolyte is pumped through the cell and, due to the electrochemical reactions, the individual concentrations of ... Read More
This tutorial example models the currents and the concentration of dissolved metal ions in a battery (corrosion cell) made from an orange and two metal nails. This type of battery is commonly used in chemistry lessons. Instead of an orange, lemons or potatoes can also be used. Read More
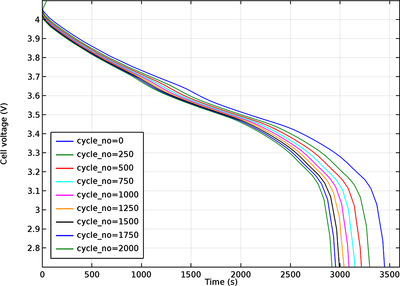
Side reactions and degradation processes may lead to a number of undesirable effects, causing capacity loss in lithium-ion batteries. Typically, aging occurs due to multiple complex phenomena and reactions that occur simultaneously at different places in the battery, and the degradation ... Read More
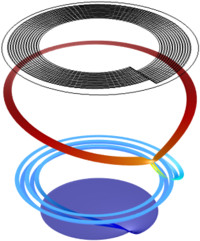
This 2D example of a vanadium flow battery demonstrates how to couple a secondary current distribution model for an ion-exchange membrane to tertiary current distribution models for two different free electrolyte compartments of a flow battery. The Ion-Exchange Membrane boundary node ... Read More
A simple equivalent circuit model approach is presented for Nickel metal hydride batteries. The 0D model consists of resistor, capacitor, current source and state-of-charge based voltage source (SOC). An Arrhenius type dependence is used to account for self-discharge. All model ... Read More
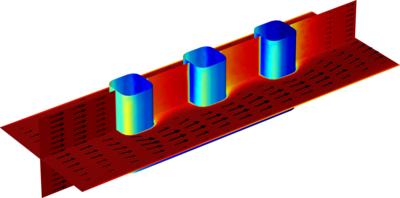
Due to abuse, such as internal or external short circuits or excessive heating, an individual battery cell may go into thermal runaway, during which the battery cell generates a significant amount of heat. If enough heat is transferred between adjacent cells during a thermal runaway ... Read More