Electrode-Electrolyte Interface Simulation in Microbubble Distillation: Role of (DDLs) as Electrochemical Cell
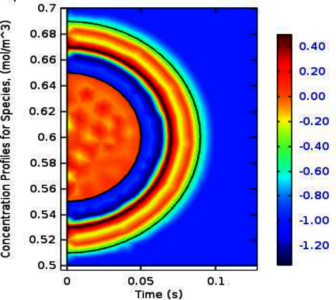
The proposed dielectric mediums of a hot microbubble interface experience different behaviors due to the electrical mobility of carriers (electron and ion) within their domains. The responses of such mediums towards the initial and average mobility of these carriers can be classified as the linear and nonlinear responses. These two kinds of response define and categorize these mediums as the linear homogeneous isotropic and nonlinear heterogeneous anisotropic dielectric mediums. Accordingly, the mediums categorization in two classes and their responses towards the mobilities of carriers motivate the approach of electrochemical phenomena within their domains. Furthermore, the restricted boundaries for these mediums are specified by the formation of dipole-double layers (DDLs) within the shell zone of this interface. Therefore, the electrochemical phenomena are implemented in COMSOL Multiphysics® for simulating (modeling) the gas-liquid interface of a hot microbubble and its shells as the electrode-electrolyte interface due to the assumed behavior of microbubble or its shells as an electrode in a specific electrolyte (the bulk of liquid).
The computational model determines the current distribution throughout these mediums (DDLs) and calculating their resistance because every electrochemical cell is already assessed by these two important factors besides its resultant voltage. This model treats the solvation process for the dissolving of air (solute) inside the bulk of liquid BTX mixture (solution). In general, the diffusion according to Nernst-Einstein relation, convection, and the independent migration of species according to the limiting molar conductivity by Kohlrausch’s law are investigated as the driving force for the transport phenomena within the imposed interface of a hot microbubble and its shells. Hence, the mass balance (mass flux) and current flux are estimated in this model by the implementation of tertiary current distribution by Nernst-Planck equation in COMSOL Multiphysics®. Furthermore, the initial and averaging cases of charge distributions within these categorized mediums are originally investigated to simulate the reaction kinetics and their effects on the mechanisms of transported species from the bulk of liquid (electrolyte) towards the surface of a hot microbubble (electrode) according to their attractiveness due to their polarity.
The results and governing equations are documented in this modeling investigation while the applications of the related factors to the electrochemical phenomena are achieved in other related models. A brief conclusion is: such a model contributes to explain other phenomena such as the black body radiation in terms of monochromatic electromagnetic radiation and visible light to excite chromophores due to the electric and magnetic field oscillations within a specific medium, or another phenomenon such as the flow of spectral energy flux density in the far field from this interface domain. Such energies are the kinetics energies result from the oscillation of electrodynamic fields. Additionally, this model can facilitate the investigating of formed (DDLs) in the separation process of organic mixtures such as the azeotropic or multicomponent mixtures through the computations of chemical potentials within (DDLs) boundaries. Finally, such a model provides evidence for ion hydration process which can prove the occurrence of electronic interactions in (DDLs) of a hot microbubble and shells.
Download
- DDLs_Paper.pdf - 0.45MB
