Modeling Thrombosis through Viscosity Variation in a Coupled CFD-Chemical Kinetics Simulation
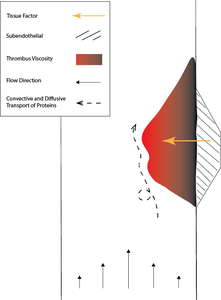
Thrombosis is the formation of a thrombus or blood clot within a vessel to maintain the integrity of the circulatory system [1]. The process involves surface interactions, with coagulation leading to the formation of thrombin and a fibrin network, and platelet aggregation [2]. Additionally, as the channel is obstructed, the rheological environment dynamically changes, adding modeling complexity [2]. Many models reduce the problem by focusing on the early stages or a single aspect of the process. This work aims to simulate the entire process from injury to a stable thrombus. The complexity will be reduced by implicitly including cellular material through a viscosity function that accounts for shear-thinning and solidification while eliminating the collision and aggregation of individual cells. The resulting model will allow us to examine the underlying mechanisms, as well as the impact of initial conditions on outcomes such as thrombus shape and clotting time.
The initial simulation is performed under physiological conditions representative of a healthy patient, and the results are validated against in vivo thrombus formation. The implementation takes advantage of the coupling of computational fluid dynamics (CFD) and chemical kinetics in COMSOL®. The Laminar Flow physics interface captures plasma flow with a user-defined viscosity function accounting for shear-thinning and solidification. The shear-thinning is approximated using the Carreau-Yasuda equation, where viscosity decays from a zero-shear value to an infinite-shear value based on the computed shear-rate. Boyd and Buick determined the equation constants for blood [3]. The result is then scaled based on the concentration of thrombin to capture solidification. If the concentration of thrombin is zero, the scaling is unity, and as the concentration grows, the scaling produces a viscosity large enough to approximate a solid.
The concentration of thrombin is determined using the Chemical Reaction Engineering Module and the Transport of Diluted Species interface. The coagulation cascade is a series of reactions involving circulating blood proteins and tissue factor bound to the surface of the subendothelial and released into the blood. It is possible to simulate all reactions in the cascade; however, to improve computational efficiency, an optimized seven species model of the coagulation cascade is implemented [4]. The circulating proteins are simulated in the bulk and replaced through inflow, while the tissue factor is introduced into the system through boundary conditions. The amount of tissue factor released by the injury site depends on the subendothelial exposure and can be varied to represent different traumas. Overall, the simulation allows variation of most inputs including the injury size, tissue factor flux, reaction rates, diffusivities, flow profile, pressure, viscosity function, and geometry.
The simulation will capture the spatiotemporal evolution of thrombosis. The varying viscosity at any one time will represent the shape and density gradient of the thrombus. At this point, the model may be used immediately or refined to include even greater molecular detail. Finally, the model’s generality and flexibility allow it to be used as a test-bed for designing experiments, studying pathologies, and evaluating treatments.