Modelling the Diffusion/Counter Diffusion of Oxygen and Solutes in Low Density Steels
Low Density Steels (LDS) mostly have additions of aluminum and/or manganese to reduce their weight and to obtain steel grades with various microstructures. The automotive industry is keenly interested in the development of LDS for reducing CO2 emissions by increasing fuel efficiency, which could make electric vehicles more desirable.
However, the introduction of alloying elements such as the manganese and aluminium, which have an even greater oxygen affinity than iron, leads to the emergence of difficult-to-remove surface oxides and complex internal (Mn, Al)O spinel-type oxides particularly influencing the ability of galvanizing (giving the finished product a protective zinc coating). Therefore, the coating ability of Fe-Mn-Al-C steels will be different from that of conventional steels. The objective of this research is to develop a fundamental understanding of the selective oxidation behavior of low density steels with high Al and Mn in annealing atmospheres which will help the investigation of the effect of the selective oxides on the coating behavior and surface quality under conditions relevant to industrial conditions.
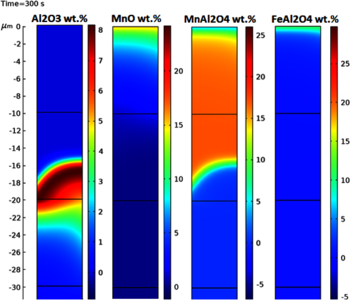
In this paper, COMSOL Multiphysics® is used to study the oxidation behavior of low density steels by the Transport of Diluted Species (Transport Phenomena) and Chemistry (Chemical Reaction Engineering) interfaces. The Transport of Diluted species interface, focuses on the diffusion of Oxygen from the surface, and solute (Al, Mn) diffusion from the geometry (bulk) which proceeds via Fick’s Laws. The Chemistry interface is the basis of the reactions taking place as the various oxygen partial pressures at the surface produce different oxides; for a high Al, Mn steel, a low partial pressure would mainly see the formation of surface and internal Al2O3 with the possibility of the spinel-type oxide MnAl2O4 also forming depending on the mole fraction of aluminum; however, with an atmosphere with high oxygen potential would see an assortment of Al2O3, MnAl2O4, FeAl2O4 oxides at various depths from the surface, with the more stable oxides forming further down where there is lower oxygen concentration.
The main portion of this model is based around the iterative process of species diffusion then subsequent reaction, the now established oxides can: block the diffusion path; speed up diffusion alongside the oxide with certain oxide morphologies which will affect the depth and composition of further reactions; and affect the rate of reaction. The finished model is expected to predict a scenario with a maximum internal oxidation for the steel, the composition of the oxides and their relative location – based on the steel composition and surface oxygen potential.
Download
- COMSOL_Conference_Poster_Final.pptx - 0.48MB
