Alkaline Electrolyzer
Application ID: 99281
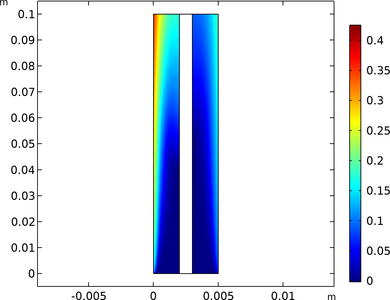
Alkaline water electrolysis is a well-established industrial process for producing hydrogen gas. In the cell, hydrogen gas is formed at the cathode whereas oxygen gas is formed at the anode.
The electrolyte is an aqueous liquid, and when the evolved gases form bubbles, the effective ionic conductivity is lowered. The generated gases may have a detrimental effect on cell performance also due to a lowered accessible surface area for the electrode reactions.
This example investigates the impact of the gas formation on the performance of an alkaline electrolysis cell.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



