Large-Scale Electrophoretic Fractionation of Rare Earths
My research group began applying microchip electrophoresis to fractionation of the rare earths more than a decade ago. The electrophoretic method we applied at microscale was isotachophoresis, which has the ability to quickly concentrate samples by several orders of magnitude while separating the analytes into a set of highly purified, contiguous zones. This technology has the advantages that, first, it is performed in a homogeneous aqueous phase without using a packing or immiscible extract phase so there is little waste. Second, only simple organic ions, e.g., sodium, chloride, acetate, lactate, etc., are need to buffer and support the solubility of the metal ions. Third, the separation mechanism depends only on applied electrical current so operating costs are low, for example, compared to chromatography.
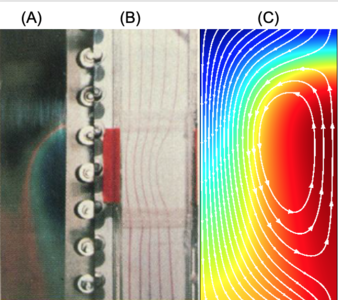
Now we are looking at strategies for scaling electrophoretic separations up to kilogram throughputs. The main impediments to scaling this class of separation technologies are (1) unstable natural convection due to unfavorable temperature gradients produced by Joule heating, (2) electrohydrodynamic instabilities due to conductivity gradients interacting with electric field gradients and (3) the very small difference in electrophoretic mobilities between many of the rare earths. My research group has developed equipment designs and operating protocols which address each of these issues. My presentation will focus on the COMSOL® modeling of these three bottlenecks and the design of a large-scale process for the electrophoretic separation of rare earths.