Modelling of cavity hydrogen pressure for a cast steel
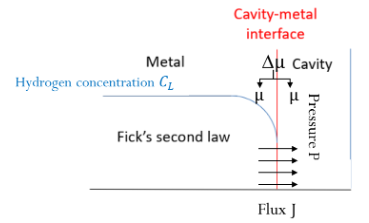
It has been well proved that hydrogen represents a harmful element for steels because it can easily embrittle them. This phenomenon is known as Hydrogen Embrittelement (H.E.) and it leads to a serious decrease in mechanical properties especially loss of ductility. The studied material is a low-alloy cast steel and it usually contains cavities which can trap hydrogen under the gaseous state. The hydrogen pressure theory is a H.E. mechanism in which atomic hydrogen H diffuses through the material and recombines to molecular hydrogen H2 inside the cavities. As a result, the internal pressure rises and it keeps rising as atomic hydrogen continues to diffuse until reaching equilibrium between the lattice hydrogen concentration and the hydrogen pressure within the cavity. Consequently, the pressure can attain hundreds of MPa in some cases and it generates a stress field around the cavity, which can lead to rupture. Unfortunately, the internal pressure cannot be measured experimentally, thus, in order to estimate it a finite element model was elaborated using COMSOL Multiphysics®. The setup consists of a rectangular metal sample that contains cavities with different dimensions. The present model is able to predict the equilibrium pressure inside a cavity based on the initial hydrogen concentration in the material. The transport of diluted species module was used to simulate the hydrogen diffusion process inside the steel. In addition, ODE and DAE interfaces module was used to calculate, at each step, the hydrogen quantity that has been absorbed by the cavity. Other equations (equation of state, fugacity…) were introduced in the model in order to try as much as possible to describe the real hydrogen behavior under high pressure. Numerical simulations have not only permitted to estimate the equilibrium pressure, but has also permitted to follow the time-dependence of hydrogen pressure during diffusion, which is very important because it allows to determine the transient maximum pressure that was reached before equilibrium. This pressure represents a crucial information that can be useful in the understanding of some H.E. cases.
Download
- Poster Comsol 2020 YAKTITI.pdf - 0.99MB



