COMSOL Events Calendar
Modeling Workflow
Electromagnetics
Structural & Acoustics
Fluid & Heat
Chemical
Interfacing
General
Show as:
Time zone:
My Time (EST)
Host Time
08:00 AM - 08:00 PM
Showing events starting between: 8 a.m. – 8 p.m.
Search
Upcoming Events
Loading Events
Jan 6
| 8:00 a.m. CET
Solving Large Models in COMSOL Multiphysics®
COMSOL
Webinar
Online

Jan 6–9
| 12:00 p.m. PST
CES
COMSOL
Exhibition
Las Vegas

Jan 13–16
| 11:00 a.m. EST
Introduction to COMSOL Multiphysics®
COMSOL
Training Course
Online
Jan 13
| 2:00 p.m. EST
Polymer Flow Modeling
COMSOL
Webinar
Online

Jan 15
| 11:00 a.m. EST
COMSOL Day: Version 6.4
COMSOL
COMSOL Day
Online
Jan 20
| 8:00 a.m. CET
The Basics of COMSOL® in 18 Minutes
COMSOL
Webinar
Online

Jan 20–23
| 11:00 a.m. EST
Battery Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online
Jan 22
| 2:00 p.m. EST
Shape and Topology Optimization with COMSOL Multiphysics®
COMSOL
Webinar
Online
Jan 27–30
| 11:00 a.m. EST
Structural Mechanics Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online

Jan 27
| 11:30 a.m. EST
The Basics of COMSOL Multiphysics® in 18 Minutes
COMSOL
Webinar
Online
Jan 29
| 2:00 p.m. EST
Simulating Optical Waveguides
Laser Focus World
Webinar
Online

Feb 3
| 8:00 a.m. CET
Topology Optimization with COMSOL® in 18 Minutes
COMSOL
Webinar
Online
Feb 10–13
| 11:00 a.m. EST
RF Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online
Feb 12
| 8:00 a.m. CET
Wie Simulation die Batterietechnologie der Zukunft formt
Process
Webinar
Online
Feb 17
| 8:00 a.m. CET
Heat Transfer Modeling with COMSOL® in 18 Minutes
COMSOL
Webinar
Online
Mar 3
| 8:00 a.m. CET
Modeling for Aerospace Engineering with COMSOL Multiphysics®
COMSOL
Webinar
Online

Mar 3–6
| 11:00 a.m. EST
Introduction to COMSOL Multiphysics®
COMSOL
Training Course
Online

Mar 3
| 2:00 p.m. EST
Editing, Repairing, and Combining Imported STL Files with CAD
COMSOL
Webinar
Online

Mar 10–13
| 11:00 a.m. EDT
CFD Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online
Mar 17
| 9:00 a.m. CET
CFD Modeling with COMSOL Multiphysics® in 18 Minutes
COMSOL
Webinar
Online
Mar 24–27
| 11:00 a.m. EDT
AC/DC Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online

Apr 14–17
| 11:00 a.m. EDT
Introduction to COMSOL Multiphysics®
COMSOL
Training Course
Online
Apr 21–24
| 11:00 a.m. EDT
Heat Transfer Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online

Apr 28–29
| 9:00 a.m. EDT
Introduction to COMSOL Multiphysics®
COMSOL
Training Course
Burlington

May 5–8
| 11:00 a.m. EDT
Acoustics Modeling in COMSOL Multiphysics®
COMSOL
Training Course
Online

May 19–22
| 11:00 a.m. EDT
Introduction to COMSOL Multiphysics®
COMSOL
Training Course
Online

Jun 23–26
| 11:00 a.m. EDT
Introduction to COMSOL Multiphysics®
COMSOL
Training Course
Online
Showing 8 of 27 events
Showing 27 of 27 events
| Date | Time | Title | Type | Host | Discipline |
|---|---|---|---|---|---|
| Jan 6 | 8:00 a.m. |
Solving Large Models in COMSOL Multiphysics® |
Webinar | COMSOL-Online |
|
| Jan 6–9 | 12:00 p.m. |
CES |
Special Event | COMSOL-Las Vegas |
|
| Jan 13–16 | 11:00 a.m. |
Introduction to COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Jan 13 | 2:00 p.m. |
Polymer Flow Modeling |
Webinar | COMSOL-Online |
|
| Jan 15 | 11:00 a.m. |
COMSOL Day: Version 6.4 |
COMSOL Day | COMSOL-Online |
|
| Jan 20 | 8:00 a.m. |
The Basics of COMSOL® in 18 Minutes |
Webinar | COMSOL-Online |
|
| Jan 20–23 | 11:00 a.m. |
Battery Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Jan 22 | 2:00 p.m. |
Shape and Topology Optimization with COMSOL Multiphysics® |
Webinar | COMSOL-Online |
|
| Jan 27–30 | 11:00 a.m. |
Structural Mechanics Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Jan 27 | 11:30 a.m. |
The Basics of COMSOL Multiphysics® in 18 Minutes |
Webinar | COMSOL-Online |
|
| Jan 29 | 2:00 p.m. |
Simulating Optical Waveguides |
Webinar | Laser Focus World - Online |
|
| Feb 3 | 8:00 a.m. |
Topology Optimization with COMSOL® in 18 Minutes |
Webinar | COMSOL-Online |
|
| Feb 10–13 | 11:00 a.m. |
RF Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Feb 12 | 8:00 a.m. |
Wie Simulation die Batterietechnologie der Zukunft formt |
Webinar | Process - Online |
|
| Feb 17 | 8:00 a.m. |
Heat Transfer Modeling with COMSOL® in 18 Minutes |
Webinar | COMSOL-Online |
|
| Mar 3 | 8:00 a.m. |
Modeling for Aerospace Engineering with COMSOL Multiphysics® |
Webinar | COMSOL-Online |
|
| Mar 3–6 | 11:00 a.m. |
Introduction to COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Mar 3 | 2:00 p.m. |
Editing, Repairing, and Combining Imported STL Files with CAD |
Webinar | COMSOL-Online |
|
| Mar 10–13 | 11:00 a.m. |
CFD Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Mar 17 | 9:00 a.m. |
CFD Modeling with COMSOL Multiphysics® in 18 Minutes |
Webinar | COMSOL-Online |
|
| Mar 24–27 | 11:00 a.m. |
AC/DC Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Apr 14–17 | 11:00 a.m. |
Introduction to COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Apr 21–24 | 11:00 a.m. |
Heat Transfer Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Apr 28–29 | 9:00 a.m. |
Introduction to COMSOL Multiphysics® |
Training Course | COMSOL-Burlington |
|
| May 5–8 | 11:00 a.m. |
Acoustics Modeling in COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| May 19–22 | 11:00 a.m. |
Introduction to COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
| Jun 23–26 | 11:00 a.m. |
Introduction to COMSOL Multiphysics® |
Training Course | COMSOL-Online |
|
Trainings by Certified Consultants
On-Demand Webinars
多物理场仿真结果的处理与可视化
Webinar
Dec 25

COMSOL Multiphysics® 6.4 版本功能简介
Webinar
Dec 18
Parameter Estimation for Nonlinear Materials
Webinar
Dec 11
Modéliser le contact mécanique avec COMSOL Multiphysics®
Webinar
Dec 11

COMSOL® 中的等离子体仿真
Webinar
Dec 11

Introducing COMSOL Multiphysics® Version 6.4
Webinar
Dec 10

Migliorare la progettazione e la gestione termica delle batterie
Webinar
Dec 10
Multiphysics Simulation of Power Transformers
Webinar
Dec 09

Modéliser les dispositifs médicaux et biomédicaux avec COMSOL Multiphysics®
Webinar
Dec 04

COMSOL® 中的化学反应工程仿真
Webinar
Dec 04

Multiphysics Simulation for Vehicle Electrification
Webinar
Dec 02
COMSOL® 多物理场仿真在新能源汽车领域中的应用
Webinar
Nov 27

Optimising Manufacturing Processes Through Simulation
Webinar
Nov 26

Simulare per il biomedicale con COMSOL Multiphysics®
Webinar
Nov 26

Designing Efficient Electric Motors with COMSOL Multiphysics
Webinar
Nov 26

使用 COMSOL® 模拟声学超材料
Webinar
Nov 20

Efficient Battery Modeling with Surrogate Models
Webinar
Nov 19

Automating Your Modeling Workflow in COMSOL Multiphysics®
Webinar
Nov 18

Modéliser le packaging et les tests de dispositifs électroniques avec COMSOL Multiphysics®
Webinar
Nov 13
使用 COMSOL 模拟 RF 和光学周期性结构
Webinar
Nov 13

The Basics of COMSOL Multiphysics® in 18 Minutes
Webinar
Nov 11

Modeling Pipe Flow & Heat in COMSOL Multiphysics®
Webinar
Nov 06

Progettare per l’industria aerospaziale con COMSOL Multiphysics®
Webinar
Nov 05

Modeling Gyroscopes and Accelerometers in COMSOL Multiphysics®
Webinar
Oct 30

COMSOL 多物理场仿真在功率器件中的应用
Webinar
Oct 23
Personalizza le tue equazioni con COMSOL Multiphysics®
Webinar
Oct 22

Modéliser les écoulements turbulents avec COMSOL Multiphysics®
Webinar
Oct 16

COMSOL 多物理场仿真在数字岩心中的应用
Webinar
Oct 16

Modeling and Simulation for Electric Motor Design
Webinar
Oct 15
Simulating Electrical Power Systems for the Grid
Webinar
Oct 14

Unravelling Chemomechanical Effects in Lithium-Ion Batteries
Webinar
Oct 09
Optimierung keramischer 3D-Drucke durch Simulation
Webinar
Oct 09

Simulare sistemi elettrici di potenza
Webinar
Oct 08

Modeling Next-Generation Battery Technologies with COMSOL Multiphysics®
Webinar
Oct 06

Modeling Emerging Battery Technologies with COMSOL Multiphysics®
Webinar
Sep 25
Modéliser le rayonnement thermique avec COMSOL Multiphysics®
Webinar
Sep 25
在 COMSOL 中创建、训练和使用代理模型
Webinar
Sep 25
Ottimizzare le simulazioni di meccanica strutturale
Webinar
Sep 24

Modeling and Simulation of MEMS Devices
Webinar
Sep 23

Elektrische Entladungen modellieren
Webinar
Sep 18

Modéliser les moteurs électriques et les générateurs avec COMSOL Multiphysics®
Webinar
Sep 18

电力设备噪声的多物理场仿真
Webinar
Sep 18
Costruire app di simulazione e digital twin con COMSOL Multiphysics®
Webinar
Sep 16
Modeling Reaction Kinetics and Pharmacokinetics with COMSOL Multiphysics®
Webinar
Sep 11
Modeling Ultrasound for Biomedical Applications
Webinar
Sep 09

Optimization of Thermal Management Systems
Webinar
Sep 03

Prediction of Electromagnetic Signatures of Naval Vessels with COMSOL Multiphysics
Webinar
Aug 28

Modellare sistemi ottici con COMSOL Multiphysics®
Webinar
Aug 28

Bonnes pratiques pour évaluer et afficher les résultats dans COMSOL Multiphysics®
Webinar
Aug 28
COMSOL 多物理场仿真在声学中的应用
Webinar
Aug 27

COMSOL 仿真 App 开发、部署和应用
Webinar
Aug 21

Modeling Room Acoustics in COMSOL Multiphysics®
Webinar
Aug 19

Modeling and Simulation for Electric Motor Design
Webinar
Aug 14

使用 COMSOL 模拟热应力
Webinar
Aug 14

Moisture Transport Modeling in COMSOL Multiphysics®
Webinar
Aug 07

COMSOL 在半导体封测中的应用
Webinar
Aug 07

Modeling Hydrogen Fuel Cells and Electrolyzers
Webinar
Aug 06

The Basics of COMSOL Multiphysics® in 18 Minutes
Webinar
Aug 05
Modeling Acoustic Metamaterials in COMSOL Multiphysics
Webinar
Jul 31
使用 COMSOL 模拟多孔介质流和地下水流
Webinar
Jul 31

Multiphysics Modeling of Power Electronics
Webinar
Jul 29

Fatigue and Durability Analysis Using COMSOL Multiphysics®
Webinar
Jul 24

Comment créer des modèles de substitution avec COMSOL Multiphysics®
Webinar
Jul 24

多物理场仿真在电力变压器中的应用
Webinar
Jul 24

Biomedical Modeling with COMSOL Multiphysics
Webinar
Jul 17

COMSOL 多物理场仿真在光学领域中的应用
Webinar
Jul 16
Understanding Liquid Metal Transport in Magnetic Fields — Simulating Magnetohydrodynamic Duct Flow
Webinar
Jul 11

Modeling Electric Motors and Drivetrains
Webinar
Jul 10

Créer des applications de simulation et des jumeaux numériques avec COMSOL Multiphysics®
Webinar
Jul 10

多物理场仿真在半导体制程中的应用
Webinar
Jul 10

COMSOL® 中的低频电磁场仿真
Webinar
Jul 03

Memristor Modeling with COMSOL Multiphysics®
Webinar
Jul 02
Modeling Fluid Mixers and Stirred Tank Reactors in COMSOL Multiphysics®
Webinar
Jun 26

使用 COMSOL 进行流固耦合仿真
Webinar
Jun 26

COMSOL® 中的传热仿真
Webinar
Jun 19

Modellierung optischer Nanostrukturen mit COMSOL Multiphysics®
Webinar
Jun 17
Underwater Acoustics
Webinar
Jun 12

Hydrogen Technology Innovation with Simulation
Webinar
Jun 12

Optimization in Structural Mechanics
Webinar
Jun 12

Modeling Hydrogen Fuel Cells and Electrolyzers
Webinar
Jun 12

Améliorer la gestion thermique des dispositifs électroniques avec COMSOL Multiphysics®
Webinar
Jun 12

多物理场仿真在先进封装中的应用
Webinar
Jun 12
Modeling Chemical Reactions and Reacting Flows Using COMSOL Multiphysics®
Webinar
Jun 05

COMSOL® 在电力设备中的应用
Webinar
Jun 05

Migliorare le tecnologie per la decarbonizzazione con la simulazione
Webinar
Jun 04

COMSOL® 网格划分功能介绍
Webinar
May 29

Introduction to Multibody Dynamics Modeling with COMSOL Multiphysics
Webinar
May 28
Automotive Acoustics Analysis Using COMSOL Multiphysics®
Webinar
May 27

Comment faire de l’optimisation et de l’estimation de paramètres avec COMSOL Multiphysics®
Webinar
May 22

Acoustics in Electronics Series, Part 4: Piezoelectric Devices
Webinar
May 20

COMSOL® 在半导体薄膜沉积工艺中的应用
Webinar
May 20
COMSOL® 中的结构力学仿真
Webinar
May 15
Analizzare i fenomeni acustici con la simulazione
Webinar
May 14

Modélisation multiphysique pour l’aéronautique et le spatial avec COMSOL Multiphysics®
Webinar
May 13

Improving Thermal Management of Batteries with COMSOL Multiphysics®
Webinar
May 09

Demokratisierung der Multiphysik-Simulation
Webinar
May 08

COMSOL® 中的 CFD 仿真
Webinar
May 08

Acoustics in Electronics Series, Part 3: Hearing Aids
Webinar
May 06

Comment utiliser le solveur temporel de COMSOL Multiphysics®
Webinar
Apr 24

将随频率变化的数据融入 COMSOL® 时域声学仿真
Webinar
Apr 24

Modéliser les Batteries avec COMSOL Multiphysics®
Webinar
Apr 17

COMSOL® 中的电池仿真
Webinar
Apr 17

Acoustics in Electronics Webinar Series, Part 2: MEMS Speakers and Microphones
Webinar
Apr 16

The Basics of COMSOL Multiphysics® in 18 Minutes
Webinar
Apr 15

Wave & Ray Optics Modeling with COMSOL®
Webinar
Apr 09

Optimization in RF and Wave Optics
Webinar
Apr 09

Modellare le scariche elettriche
Webinar
Apr 09

Meshing Your Models in COMSOL Multiphysics®
Webinar
Apr 08

Multiphysics Simulation in Semiconductor Packaging
Webinar
Apr 03

使用 COMSOL® 模拟生物传感器和检测设备
Webinar
Mar 27

Acoustics in Electronics Webinar Series, Part 1: Loudspeakers
Webinar
Mar 25

Esperienze di simulazione nell'additive manufacturing: dal design al processo
Webinar
Mar 20
Simulating Electrical Power Systems for the Grid
Webinar
Mar 20

Comment Utiliser Efficacement les Études Stationnaires de COMSOL Multiphysics®
Webinar
Mar 20
Modellierung biomedizinischer Geräte mit COMSOL Multiphysics®
Webinar
Mar 20
Multiphysics Modeling of Electronic Components
Webinar
Mar 20

使用 COMSOL® 模拟电声器件
Webinar
Mar 20

Using Geometry Modeling Tools in COMSOL Multiphysics®
Webinar
Mar 18

Improving Thermal Management of Batteries with COMSOL Multiphysics®
Webinar
Mar 18

Modéliser les Composants Électroniques avec COMSOL Multiphysics®
Webinar
Mar 13

Modeling Chemical Reactions and Reacting Flows Using COMSOL Multiphysics®
Webinar
Mar 06

使用 COMSOL® 进行锂离子电池模型的参数估计
Webinar
Mar 06

Integrating Frequency-Dependent Data into Time-Domain Acoustics Analysis
Webinar
Feb 27

使用 COMSOL® 模拟放电现象
Webinar
Feb 27
Introduction to Uncertainty Quantification
Webinar
Feb 26

Entwicklung von Leistungselektronik und Kabelsystemen
Webinar
Feb 25
使用 COMSOL® 模拟雷达和天线
Webinar
Feb 20

Efficient Battery Modeling with Surrogate Models
Webinar
Feb 18
Comment construire un maillage dans COMSOL Multiphysics®
Webinar
Feb 18

The Basics of COMSOL Multiphysics® in 18 Minutes
Webinar
Feb 13

Modéliser la Corrosion et les Systèmes de Protection contre la Corrosion avec COMSOL Multiphysics®
Webinar
Feb 13
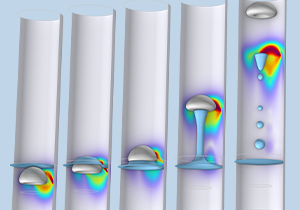
Designing a Magnetorheological-Fluid-Based Clutch Using COMSOL Multiphysics®
Webinar
Feb 13

使用模型管理器进行仿真数据管理
Webinar
Feb 13

Innovare le tecnologie dell'idrogeno con la simulazione
Webinar
Feb 11

Simulare il comportamento termico di dispositivi e processi
Webinar
Jan 30

Modeling Biochemical Sensors and Testing Devices with COMSOL®
Webinar
Jan 29

Modeling Optical Nanostructures with COMSOL Multiphysics®
Webinar
Jan 23

Améliorer les Performances des Piles à Combustible et des Électrolyseurs avec COMSOL Multiphysics®
Webinar
Jan 23

Electric Discharge Modeling
Webinar
Jan 21

Automotive Acoustics Analysis Using COMSOL Multiphysics®
Webinar
Jan 16

The Basics of COMSOL Multiphysics® in 18 Minutes
Webinar
Jan 14
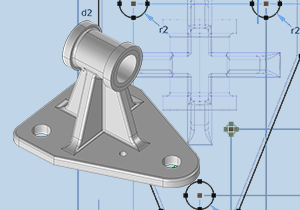
Comment Construire efficacement sa Géométrie avec le Module Design
Webinar
Jan 14

Tutte le novità di COMSOL Multiphysics® 6.3
Webinar
Jan 10

LiveLink™ for MATLAB® 和 LiveLink™ for Simulink® 功能简介
Webinar
Jan 09

COMSOL® 中的低频电磁场仿真
Webinar
Jan 03

使用 COMSOL Multiphysics® 优化设计
Webinar
Dec 26

COMSOL® 中的结构力学仿真
Webinar
Dec 19

COMSOL® 中的传热仿真
Webinar
Dec 12

COMSOL® 中的多相流仿真
Webinar
Dec 05

COMSOL Multiphysics® 6.3 版本功能简介
Webinar
Nov 28

Introducción a COMSOL Multiphysics® en 18 Minutos
Webinar
Nov 26
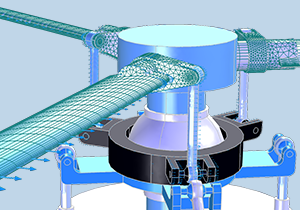
COMSOL® 中的转子动力学和多体动力学仿真
Webinar
Nov 21

COMSOL® 求解器介绍及设置方法
Webinar
Nov 14
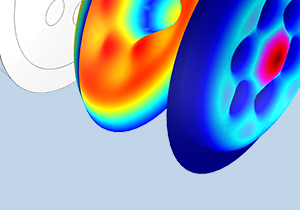
使用 COMSOL Multiphysics® 模拟声学超材料
Webinar
Nov 07
多物理场仿真结果的处理与可视化
Webinar
Oct 24

COMSOL® 中的等离子体仿真
Webinar
Oct 17
COMSOL® 网格划分功能介绍
Webinar
Oct 10

电力变压器的多物理场仿真
Webinar
Sep 19

使用 COMSOL Multiphysics® 仿真高性能电机
Webinar
Sep 12

不确定性量化在多物理场仿真中的应用
Webinar
Sep 05

超声波及其应用的多物理场仿真
Webinar
Aug 29

Modeling Hydrogen Production, Storage and Utilization
Webinar
Aug 21
使用 COMSOL Multiphysics® 模拟光电半导体
Webinar
Aug 15

COMSOL® 多物理场仿真加速数字岩心技术发展
Webinar
Aug 08

使用代理模型开发高效的 COMSOL® 仿真 App
Webinar
Aug 01

COMSOL® 多物理场仿真在地热能开发中的应用
Webinar
Jul 25

COMSOL® 仿真 App 开发、部署和应用
Webinar
Jul 18
COMSOL® 中的光学仿真
Webinar
Jul 11
非线性结构材料参数估计
Webinar
Jul 04

基于 COMSOL® 构建电力设备的数字孪生
Webinar
Jun 27
COMSOL® 室内声学仿真
Webinar
Jun 20

COMSOL Multiphysics® 在半导体制造中的应用
Webinar
Jun 13

COMSOL Multiphysics® 在绿氢技术中的应用
Webinar
Jun 06

使用 COMSOL Multiphysics® 模拟放电现象
Webinar
May 28
使用 COMSOL® 模拟 SAW/BAW 器件
Webinar
May 16
COMSOL® 中的换热器仿真
Webinar
May 09
COMSOL Multiphysics® 中的流固耦合仿真
Webinar
Apr 18
多尺度电磁波仿真
Webinar
Apr 11

COMSOL® 微执行器和微型电机仿真
Webinar
Apr 02

COMSOL® 中的湍流仿真
Webinar
Mar 26

COMSOL® 仿真在电力电子技术中的应用
Webinar
Mar 14

COMSOL® 中的流体动压轴承仿真
Webinar
Mar 12
使用 COMSOL Multiphysics® 模拟 EMI/EMC 现象
Webinar
Mar 07
COMSOL® 中的热辐射仿真
Webinar
Mar 05
使用 COMSOL Multiphysics® 模拟 MEMS 加速度计和陀螺仪
Webinar
Feb 29

COMSOL® 中的扬声器和麦克风仿真
Webinar
Jan 04

Introdução ao COMSOL Multiphysics® em 18 minutos
Webinar
Jun 13
Showing 6 of 187 events
Showing 187 of 187 events
Trainings by Certified Consultants
- Product Information
- Products
- Specification Chart
- License Options
- System Requirements
- Release History
- Support and Services
- Support Center
- My Support Cases
- Knowledge Base
- Partners and Consultants
- Documentation
- Product Download
- Company
- About
- Careers
- Press
- Contact Us
- |
- Privacy Policy
- |
- Trademarks
- |
- Cookie Settings
- © 2025 by COMSOL. All rights reserved
