Aluminum Anodization
Application ID: 49061
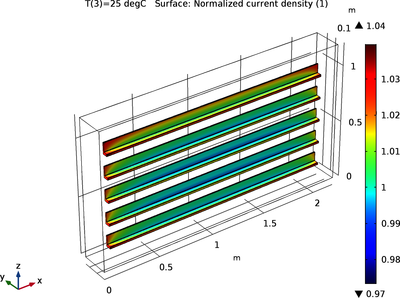
When anodizing aluminum, the surface is electrochemically altered to form an abrasive and corrosion-resistive Al2O3 film. The electrode kinetics during the process are only marginally affected as the oxide layer grows, so a stationary analysis of the current distribution is sufficient to determine the uniformity of this layer’s thickness.
Anode kinetics from experimental polarization data are used to investigate the uniformity of the anodization current density at three different temperatures, for a specified average current density. At higher temperatures, the cell potential is reduced while the current distribution and thickness become less uniform. This is attributed to lower activation losses (faster kinetics) at higher temperatures.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Battery Design Module, Corrosion Module, Electrochemistry Module, Electrodeposition Module, or Fuel Cell & Electrolyzer Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



