Electroanalytical Methods
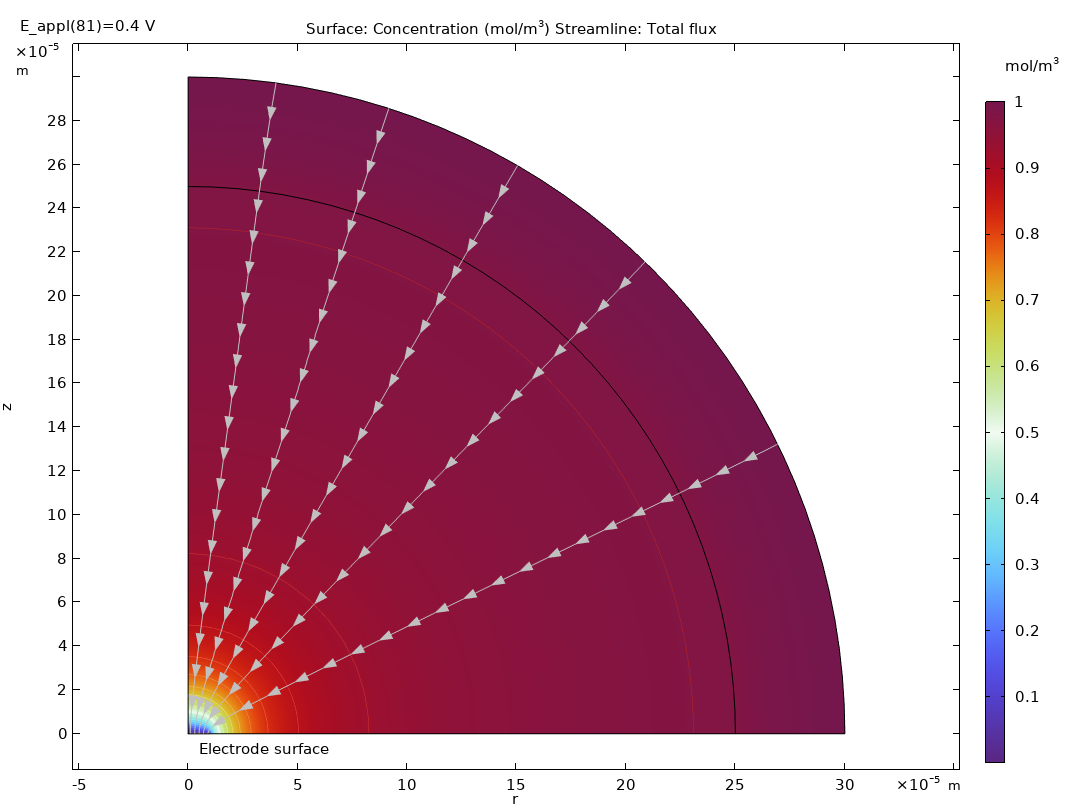
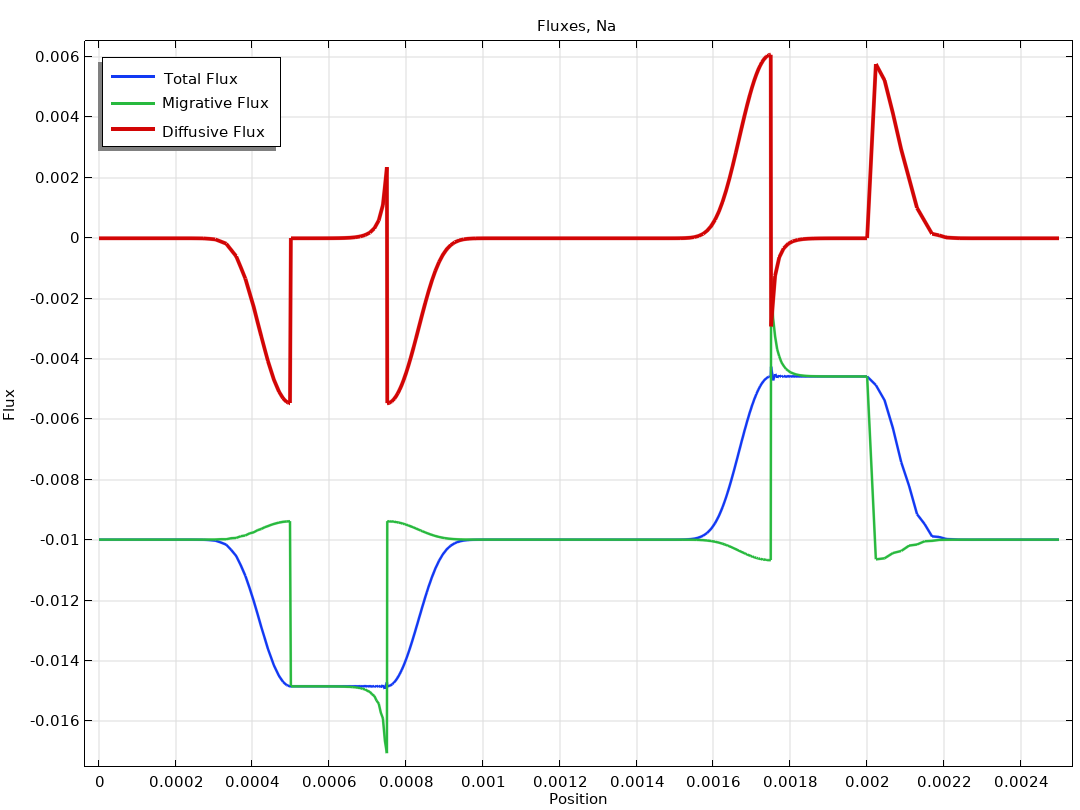
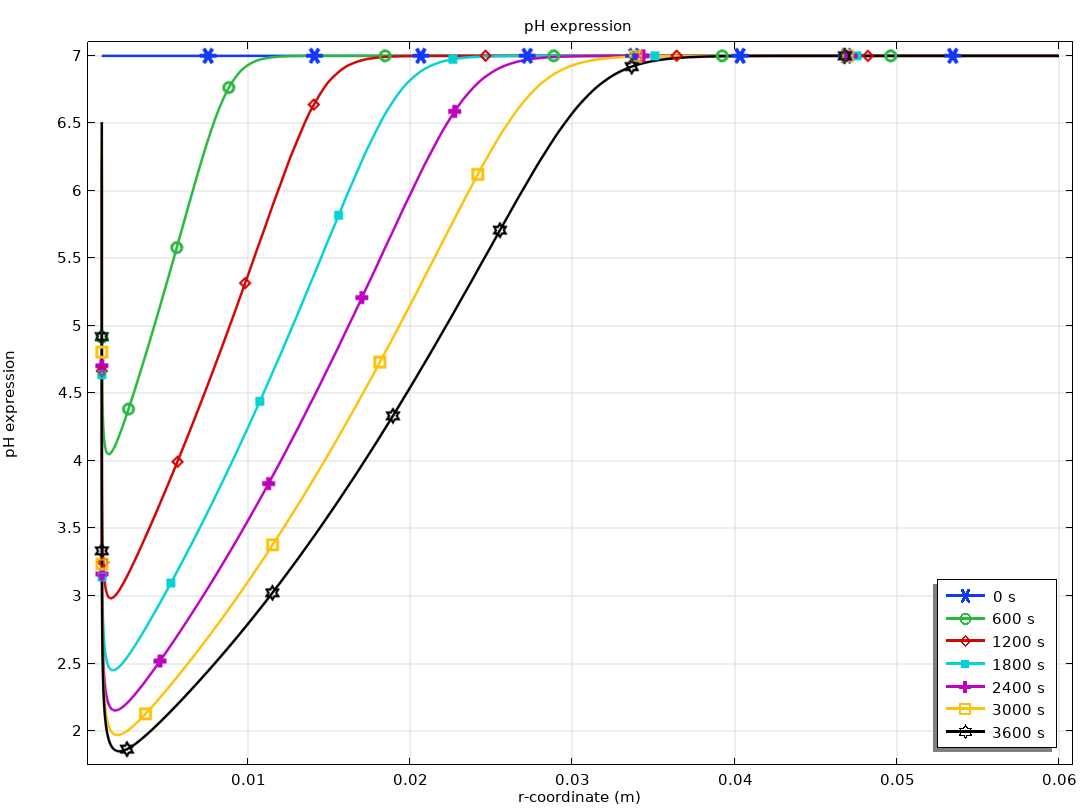
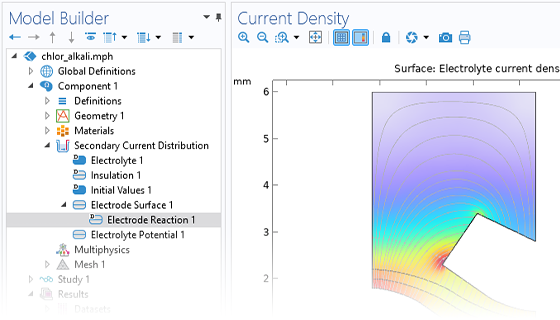
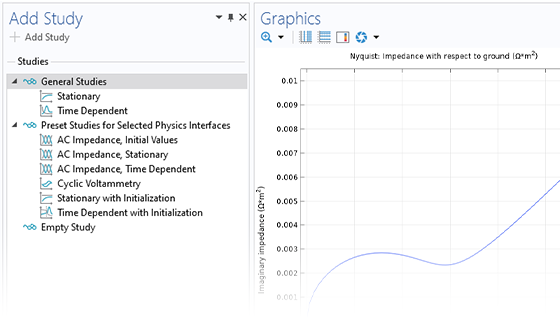
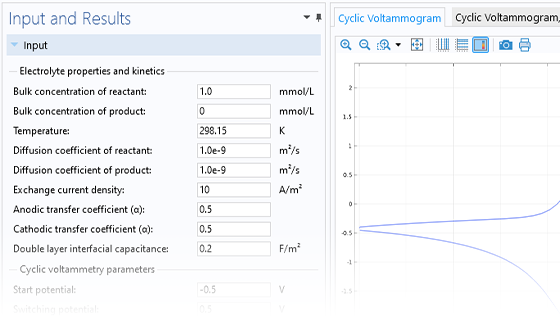
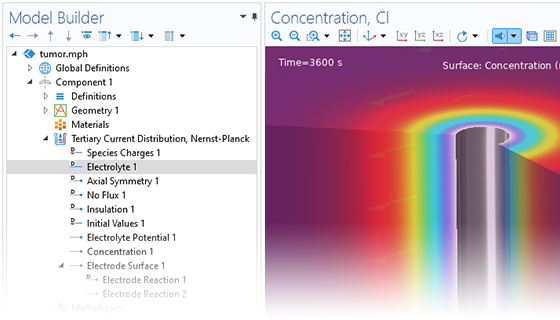
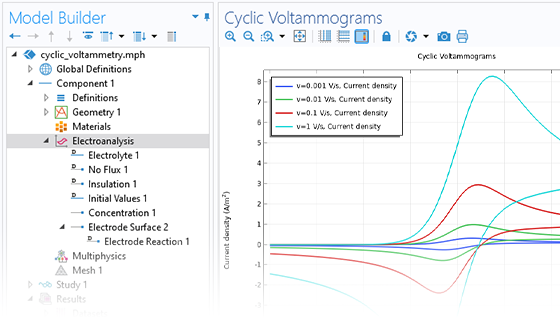
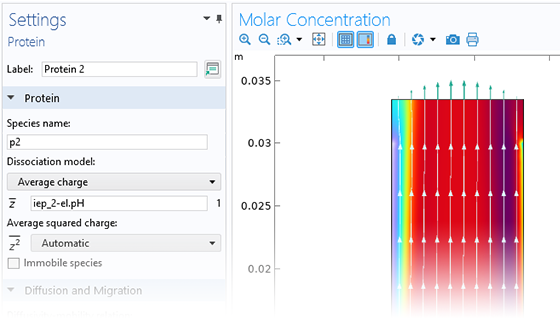
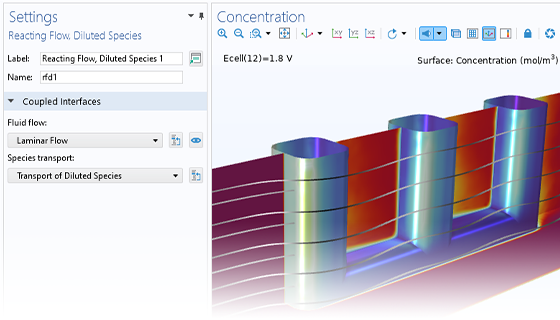
The Electrochemistry Module is able to describe common electroanalytical methods, including (cyclic) voltammetry, (chrono) amperometry, potentiometry, coulometry, and electrochemical impedance spectroscopy (EIS). These methods can be used for modeling and investigation in either a static electrolyte solution or in an electrolyte solution subject to a forced fluid flow. By using the Electrochemistry Module together with the Optimization Module, users can determine properties from the combined experimental and simulation results, such as exchange current densities, charge transfer coefficients, specific active surface areas, diffusivities, and reaction mechanisms. These can subsequently be used in industrial applications for accurate modeling and design optimization.